Registrieren Sie Ihre Medizinprodukte:
weltweit, sicher und valide.
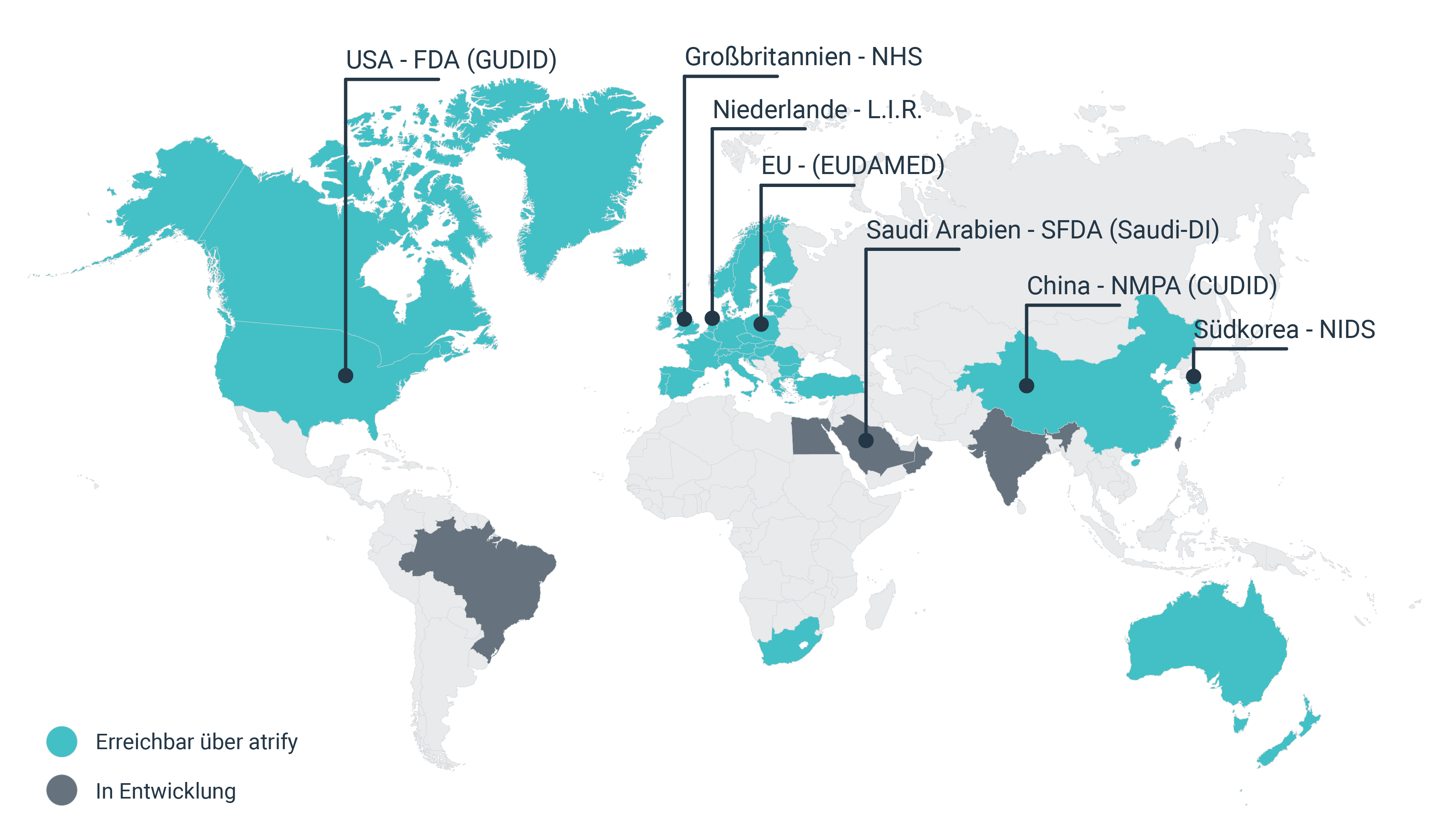
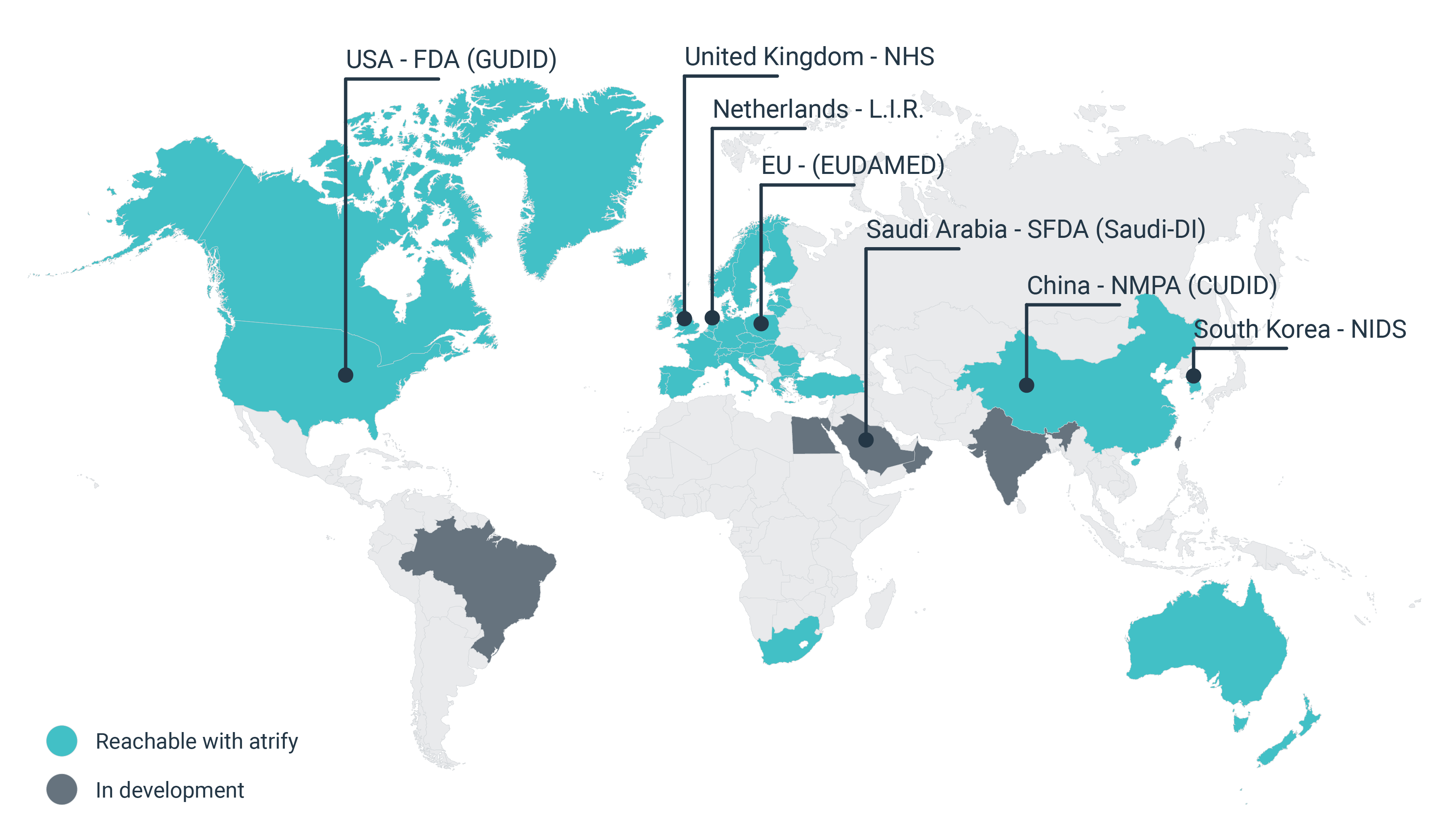
Der UDI Standard dient der weltweit einheitlichen Kennzeichnung von Medizinprodukten und soll eine höhere Markttransparenz, eine bessere Rückverfolgbarkeit und Patientensicherheit gewährleisten.
Mit uns setzen Sie die Anforderungen erfolgreich um.

Register your medical devices:
worldwide, safe and valid.
UDI standards are supporting globally uniform labeling of medical devices and are intended to ensure greater market transparency, better traceability and increased patient safety.
We are happy to work with you to implement the requirements for both commercial and regulatory use.





Our modules
Regulatory databases and authorities
Commercial partners

UK (NHS)

GPOs, clinics and hospitals (via GDSN)
GOOD PREPARATION
“Many customers simply follow the roadmap of the respective databases, but detailed and extensive preparation should not be underestimated”
We have reached a real milestone with the introduction of our service plans. Our customers are consistently delighted with the scope and flexibility of our services. In addition, we make it easier for our customers to plan their budgets thanks to the firmly calculable package sizes. A true all-round carefree package.
GUTE VORBEREITUNG
“Viele Kunden folgen einfach der Roadmap der jeweiligen Datenbanken, aber eine detaillierte und umfangreiche Vorbereitung ist nicht zu unterschätzen.”
Mit der Einführung unserer Servicepläne haben wir einen echten Meilenstein erreicht. Unsere Kunden sind durchweg begeistert vom Umfang und der Flexibilität unserer Leistungen. Darüber hinaus erleichtern wir unseren Kunden die Budgetplanung durch fest kalkulierbare Paketgrößen. Ein echtes Rundum-Sorglos-Paket.

Lionel Tussau
Healthcare Market Unit - Global Lead
Free EUDAMED Online Session
Send your data the way YOU want
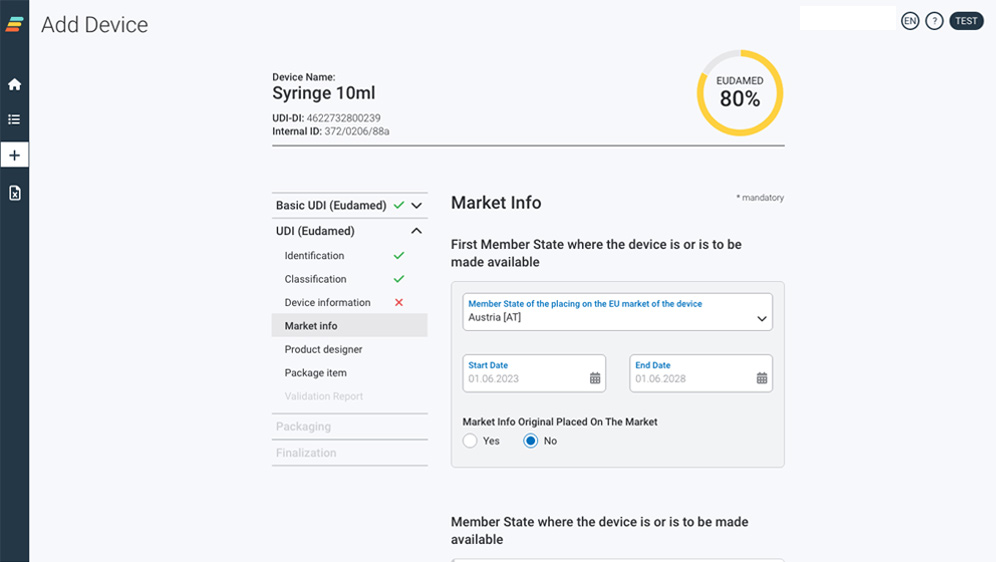
Our user interface for manual data capturing
Intuitive & state of the art.
Enter your data manually into the self-explanatory application in the web browser or upload your UDI data conveniently via Excel-sheet.
Automatic data exchange
High-performance & reliable.
With the automatic M2M (machine to machine) connection, your data is sent quickly, completely and securely from your in-house system via atrify to the respective data recipient.

Senden Sie Ihre Daten so, wie SIE es möchten
Unsere Benutzeroberfläche für die manuelle Datenerfassung
Intuitiv & modern.
Geben Sie Ihre Daten manuell in unser intuitives Anwendungsprogramm im Webbrowser ein oder laden Sie Ihre UDI Daten bequem per Excel-Tabelle hoch.
Der automatische Datenaustausch
Leistungsfähig & zuverlässig.
Mit der automatischen M2M (machine to machine) Anbindung, werden Ihre Daten schnell, vollständig und sicher von Ihrem hauseigenen System zum jeweiligen Datenempfänger gesendet.

Our Healthcare Services
With our services, you can master any UDI challenge. We support you from the analysis of each UDI Registry’s attribute requirements and the development of the appropriate strategy, to your implementation into the atrify UDI Solution, and successful registration of your medical device products.
Advisory Services
Trainings
Premium Service
Service Plans
GAP (Attribute) Analysis
Implementation and Testing Services
Unsere Healthcare Services
Mit unseren Services meistern Sie jede UDI-Challenge. Wir unterstützen Sie von der Analyse der Attributanforderungen des jeweiligen UDI-Registers über die Entwicklung der passenden Strategie bis hin zur Implementierung in die atrify UDI Solution und der erfolgreichen Registrierung Ihrer Medizinprodukte.
Advisory Services
Trainings
Premium Services
Service Plans
GAP (Attribut) Analyse
Implementierung und Testung
Steigern Sie Ihren Registrierungserfolg mit unserem Support
Unser umfassender Support gewährleistet einen nahtlosen Prozess, die Einhaltung der Vorschriften und einen größeren Erfolg bei der Registrierung von Medizinprodukten.
Elevate your registration success with expert support
Our comprehensive support ensures a seamless process, compliance, and elevated success foryour medical device registration.
Comprehensive guide
Meet UDI requirements and navigate complex documentation with expert help
Mastering complexity
Timely responses, risk mitigation, and understanding of "undocumented validations".
Leverage global data models
Simplify enrollment, gain insights, and achieve faster, higher quality results
Unparalleled support
Access UDI experts, resolve issues faster, and stay informed through our industry connections.
Learn from our UDI experts
atrify healthcare eBooks
With our EUDAMED and GUDID eBooks you will be well prepared for UDI registration in Europe and the US!
Online Sessions
In our free online sessions, we have an intensive exchange with experts, customers and partners on the topic of UDI, solutions, Eudamed, etc.
Profitieren Sie von unserer UDI Expertise
atrify Healthcare eBooks
Mit unseren aktuellen EUDAMED und GUDID eBooks werden sie bestens auf die UDI Registrierung in Europa und den USA vorbereitet!
Online Sessions
In unseren kostenfreien Online Sessions tauschen wir uns intensiv mit Experten, Kunden und Partnern zum Thema UDI, Lösungen, EUDAMED etc. aus.
atrify – ein Partner,
auf den Sie sich verlassen können

Jahrzehntelange Erfahrung für Sicherheit und Vertrauen
Durch langjährige Mitarbeit in Gremien von Behörden und Verbänden gestalten wir Entscheidungen zum Thema UDI aktiv mit. Informationssicherheit ist dabei von größter Bedeutung.
- ISO 27001 zertifiziert
- Co-Vorsitz der EUDAMED IT Expert Group (MedTech Europe)
- Enge Zusammenarbeit mit der Europäischen Kommission
- GS1 Global Healthcare Leadership Mitglied
Wir unterstützen Sie bei jedem Schritt
Ganz egal, in welcher Phase der UDI Registrierung Sie sich befinden, wir helfen Ihnen Ihr Ziel schnellstmöglich zu erreichen – von der Analyse bis zur erfolgreichen Registrierung.
- Mit unseren Experten zur optimale Strategie für Ihre UDI Registrierung
- Unterstützende Services vor, während und nach der Registrierung
- Unser Support-Team unterstützt Sie bei allen Fragestellungen
atrify – a partner you can rely on

Decades of experience for security and trust
Thanks to our many years of involvement in committees of authorities and associations, we play an active role in shaping decisions on the subject of UDI. Information security is of paramount importance.
- ISO 27001 certified
- Co-chair of the EUDAMED IT Expert Group (MedTech Europe)
- Close cooperation with the European Commission
- GS1 Global Healthcare Leadership Member
We support you every step of the way
No matter what stage of UDI registration you are in, we will help you achieve your goal as quickly as possible – from analysis to successful registration by:
- Optimizing your strategic approach to UDI registration
- Supporting your team before, during and after registration process
- Providing solutions that fit your long term UDI registration needs
Healthcare Blog
Summer break - Welcome to the last UDI playground of EUDAMED
Healthcare manufacturers will have 1 more chance to participate in the EUDAMED playground for UDI & Device registration module which will be opened by the European Commission at the end of July...
@GP and atrify - Joint Online Session for the French Community
As an ongoing effort to provide information on regulations, atrify hosts online sessions in multiple languages on EUDAMED, UDI regulations, and the exchange of product content between trading...
The European MDR is live!
On 26th May 2021, the EU Medical Devices Regulation (MDR) finally came into full application, superseding the former medical device directives from the 1990's. EUDAMED has been live since December...
Updates in the Healthcare Industry
There are a number of regulatory changes in healthcare at the moment that businesses need to keep a close eye on - and we're here to help. Firstly, changes to EUDAMED deadlines for...
@GP and atrify - a partnership that benefits us all
We are pleased to announce the new partnership between atrify and @GP. The French market will therefore benefit from the best of both solutions and expertise - atrify having built a successful...
EUDAMED - The countdown is on! Making the leap from GUDID to EUDAMED
EUDAMED is getting closer every day and testing is about to restart for the last UDI registration playground before going live in a couple of months. Time is getting short for companies to get their...